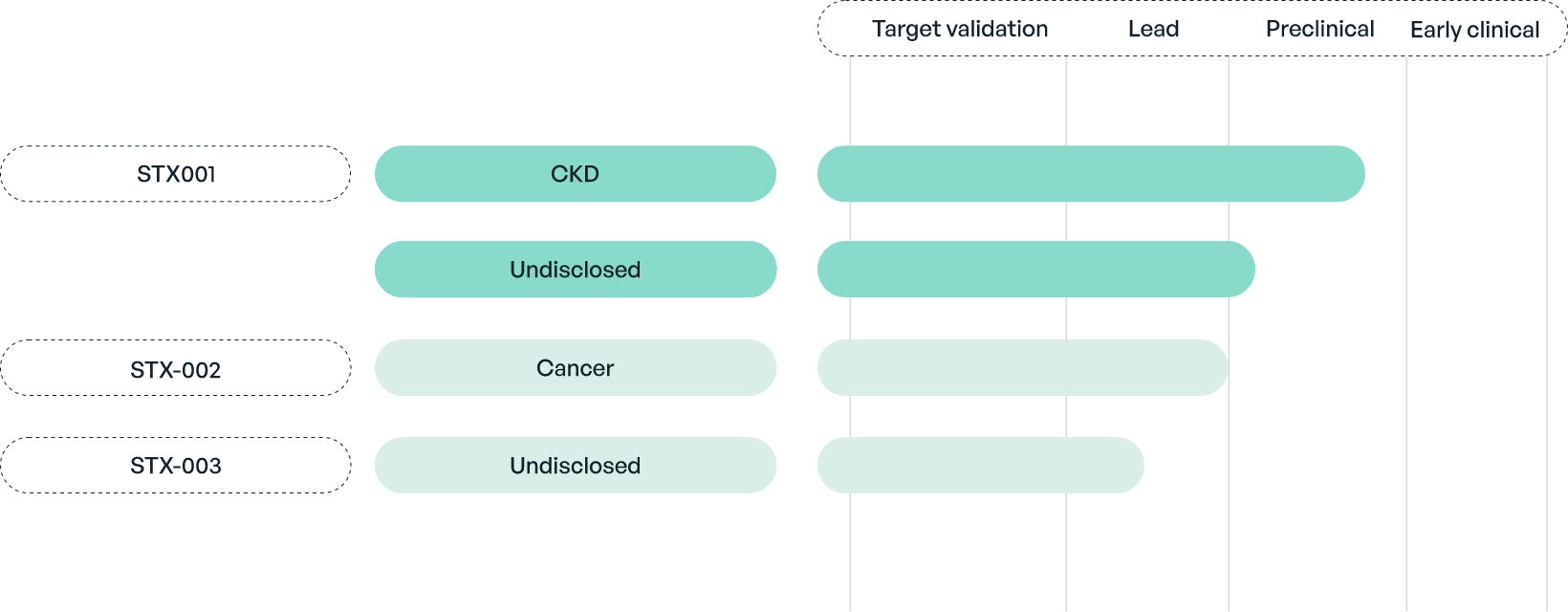
Senya Therapeutics is developing proprietary biologics targeting LRG1. The lead compound, STX-001 (Magacizumab), is a fully humanised function-blocking antibody against LRG1 isolated and developed from an original panel of more than 100 mouse monoclonals.
STX-001 is being developed to treat chronic kidney disease and an undisclosed fibrotic disease. It has shown good efficacy and safety in several preclinical models. Despite its overall excellent therapeutic profile, Senya is currently working on potential enhancements.
In addition to STX-001, Senya is developing several additional anti-LRG1 antibodies.
Senya Is Developing An Extensive Portfolio Of Anti-LRG1 Therapeutics